How To Calculate Concentration Of Weak Acid From Titration Curve . as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause.
from general.chemistrysteps.com
by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause. the concentration of an acid solution can be determined by. for the titration of a weak acid with a strong base, the ph curve is initially.
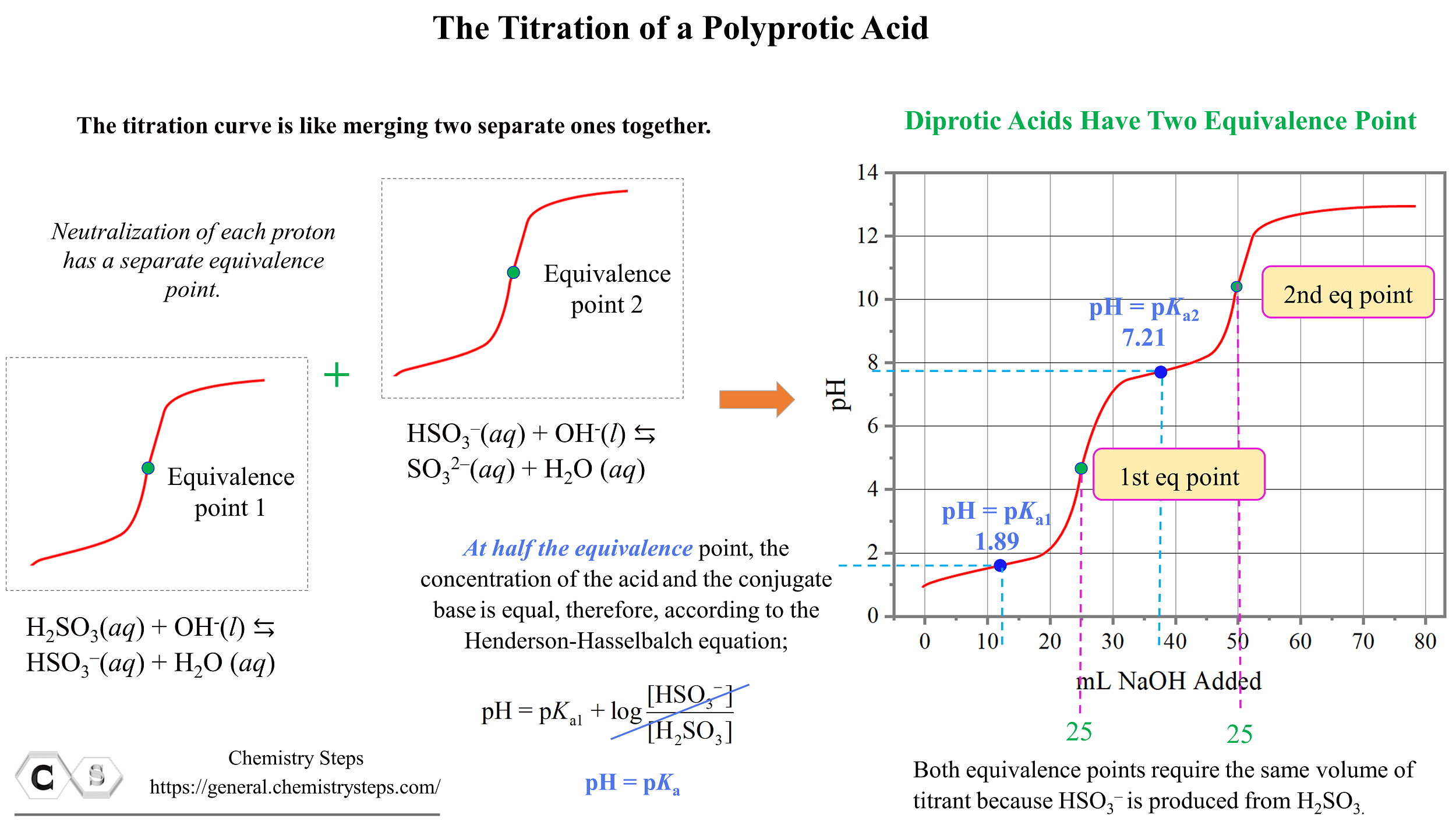
Titration of a Polyprotic Acids Chemistry Steps
How To Calculate Concentration Of Weak Acid From Titration Curve as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. the concentration of an acid solution can be determined by. by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the.
From socratic.org
Why do we use indicators in acidbase titrations? Socratic How To Calculate Concentration Of Weak Acid From Titration Curve Since an acid and its conjugate base are in equilibrium we can. by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause. for the titration of a weak acid with a strong base, the ph curve is initially. the concentration of an acid solution can. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. the concentration of an acid solution can be determined by.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. for. How To Calculate Concentration Of Weak Acid From Titration Curve.
From chemistry.stackexchange.com
How would a pH curve look like for titration of diluted weak base How To Calculate Concentration Of Weak Acid From Titration Curve as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. the concentration of an acid solution can be determined by. by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause. Since an acid and its. How To Calculate Concentration Of Weak Acid From Titration Curve.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts How To Calculate Concentration Of Weak Acid From Titration Curve the concentration of an acid solution can be determined by. for the titration of a weak acid with a strong base, the ph curve is initially. Since an acid and its conjugate base are in equilibrium we can.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From courses.lumenlearning.com
AcidBase Titrations Chemistry How To Calculate Concentration Of Weak Acid From Titration Curve by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause. for the titration of a weak acid with a strong base, the ph curve is initially.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. as explained discussed, if we know \(k_a\) or \(k_b\) and. How To Calculate Concentration Of Weak Acid From Titration Curve.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax How To Calculate Concentration Of Weak Acid From Titration Curveif you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. Since an acid and its conjugate base are in equilibrium we can. Web. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
Polyprotic Acids & Bases Titration Curves YouTube How To Calculate Concentration Of Weak Acid From Titration Curve as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. Since an acid and its conjugate base are in equilibrium we can. for the titration of a weak acid with a strong base, the ph curve is initially. by adding either an acid or a base with. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate Concentration Of Weak Acid From Titration Curve for the titration of a weak acid with a strong base, the ph curve is initially. as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. Since an acid and its conjugate base are in equilibrium we can. by adding either an acid or a base with. How To Calculate Concentration Of Weak Acid From Titration Curve.
From saylordotorg.github.io
AcidBase Titrations How To Calculate Concentration Of Weak Acid From Titration Curve as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. the concentration of an acid solution can be determined by. for the titration of a weak acid with a strong base, the ph curve is initially. Since an acid and its conjugate base are in equilibrium we. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.writework.com
Titration of amino acids WriteWork How To Calculate Concentration Of Weak Acid From Titration Curve by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause. as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. the concentration of an acid solution can be determined by. Since an acid and its. How To Calculate Concentration Of Weak Acid From Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts How To Calculate Concentration Of Weak Acid From Titration Curve the concentration of an acid solution can be determined by. by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause. as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak.if you only have. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.vrogue.co
Figure 10 11 Strong Acid And Weak Base Titration Curv vrogue.co How To Calculate Concentration Of Weak Acid From Titration Curve the concentration of an acid solution can be determined by. Since an acid and its conjugate base are in equilibrium we can.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. by adding either an acid or a base with a. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.riset.guru.pubiway.com
Titration Curves Of Strong And Weak Acids And Bases Vernier Riset How To Calculate Concentration Of Weak Acid From Titration Curve for the titration of a weak acid with a strong base, the ph curve is initially. as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. Since an acid and its conjugate base are in equilibrium we can. the concentration of an acid solution can be determined. How To Calculate Concentration Of Weak Acid From Titration Curve.
From philschatz.com
AcidBase Titrations · Chemistry How To Calculate Concentration Of Weak Acid From Titration Curveif you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. as explained discussed, if we know \(k_a\) or \(k_b\) and the initial concentration of a weak acid or a weak. the concentration of an acid solution can be determined by. Since an. How To Calculate Concentration Of Weak Acid From Titration Curve.
From mavink.com
Strong Acid And Strong Base Titration Curve How To Calculate Concentration Of Weak Acid From Titration Curve by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to cause.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. the concentration of an acid solution can be determined by.. How To Calculate Concentration Of Weak Acid From Titration Curve.
From socratic.org
A "25.0mL" sample of "0.150mol L"^(1) acetic acid is titrated with a How To Calculate Concentration Of Weak Acid From Titration Curveif you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. Since an acid and its conjugate base are in equilibrium we can. by adding either an acid or a base with a known molarity (the titrant) and measuring how much is needed to. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
Titration of a strong acid with a strong base Chemistry Khan How To Calculate Concentration Of Weak Acid From Titration Curve the concentration of an acid solution can be determined by.if you only have a weak acid or base with water left (which occurs in weak/strong titrations at the equivalence point), you must calculate the. Since an acid and its conjugate base are in equilibrium we can. as explained discussed, if we know \(k_a\) or \(k_b\) and. How To Calculate Concentration Of Weak Acid From Titration Curve.